How plague attacks us – and how we can defend ourselves
The plague that is believed to have caused the Black Death still occasionally ravages populations, albeit to a much smaller extent than before. Now we know more about how the bacteria attack us.
BUBONIC PLAGUE: Thousands of people around the world may be affected by plague every year. The bacterium Yersinia pestis causes the disease, and was also responsible for the Black Death that killed tens of millions of people in the mid-1300s.
But how does the plague bacterium attack human beings, and how does the body defend against its constantly evolving attacks? New research on the bacterium is yielding new answers.

Theodor Kittelsen produced a series of drawings on the theme of the Black Death. The plague was an old woman who walked around from house to house. This drawing was called “Mor, der kommer en kjerring,” which translated means, “Mother, an old woman is coming this way.”
An “arms race”
Evolution seems to work like an “arms race” between bacteria and humans.
Bacteria attack the human body in new ways, but over several generations, human beings become better at fighting them and build resistance to the attacks – at least until the bacteria find new and different means of attacking us.
To put it another way, bacteria find ways to inhibit immune responses, but the body also develops new ways to circumvent the blockade and to recognize the infection. “We can only hope that humans win in the end!” says Egil Lien, an adjunct professor in NTNU’s Department of Cancer Research and Molecular Medicine.
Lien is part of a group at the NTNU-CEMIR research centre, which among other things investigates how bacteria attack people. For this project, Lien’s group researched bacterial effector proteins.
- You might also like: The Black Death bacteria continues to kill
Confuses human cells
Effector proteins are signal substances from bacteria that trigger a particular reaction when they enter a host cell. Many bacteria that cause disease typically use specialized secretion systems to introduce their effector proteins into human cells.
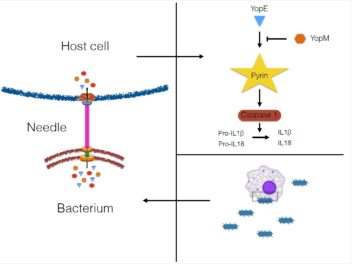
Researchers focused on how bacteria manipulate the cell’s secretion of cytokines (or signal molecules) IL-1β and IL-18. This type of excretion can produce anti-bacterial responses in mammals. Illustration: Pontus Ørning, NTNU-CEMIR
These effector proteins can have various functions, but their main purpose is to provide a good growing environment for the invading bacteria.
Bacteria can stop attacks and bacteria-inhibiting activities on themselves by blocking the host cells’ immune responses, which is often the task of the innate immune system.
Put simply, bacteria can use the effector proteins to create chaos inside cells, allowing the infection to spread without being effectively stopped.
Multiple methods
Bacteria can secrete effector proteins in several ways. One of the best-known methods is the type 3 secretion system, which is found in various disease-causing pathogens such as salmonella, E. coli, shigella and Yersinia.
“These secretion systems are sophisticated nanomachines,” says Lien. “They form a ‘needle’ from the bacterium and create a pore in the target cell membrane that the effector proteins are released into.”
Researchers associated with NTNU-CEMIR use Yersinia pestis – that is, the bacterium that causes bubonic plague – as a model system that demonstrates how several types of bacteria work.
The Black Death was most likely a combination of the bubonic and pneumonic plagues, according to most scientists in the field.
Plague on the stairs

Eyes deep in
the corpse skull,
rolling, running,
they squint and sting
sharp as awls,
glowing and seeing in
the dark like a cat.
Plague sweeps through
each and every nook.
Stop the raking
sweep, sweep!
Time is short
all must go
Peter and Paul,
beginning to end.
Plague likes it
weather is good,
sad and dark.
Broom sweeps through
in nooks and crooks.
All is sad,
wonderfully sad.
Death and the dead,
stench and
decay.
(Pesta kommer. Th. Kittelsen)
Bacteria control cytokines
Researchers at NTNU-CEMIR have discovered how some of these bacterial effector proteins can block new signalling pathways in our innate immune system.
The researchers focused on how bacteria manipulate the cell’s secretion of the signal molecules (called cytokines) IL-1β and IL-18. These excretions can produce anti-bacterial responses in mammals.
“Yersinia is very effective in controlling the secretion of these cytokines. One type of effector molecule called YopM can inhibit the activation of a cell complex called the pyrin inflammasome, which regulates the release of IL-1β and IL-18,” says Lien.
One bacterial strain that lacks YopM and that is weakened in normal mice can cause disease reactions in mice lacking pyrin. This result has proven that this mechanism is important for immune functions in mammals.
New defence mechanisms
Evolution allows humans can develop new defence mechanisms against such attacks. People who survive bacterial attacks and go on to have children will pass on their genes and their ability to resist disease to the next generation.
Eventually, individuals who fight off bacterial attacks can come to predominate in an entire population, especially if the illness is so damaging or deadly that it prevents people from passing on their genes.
This situation can lead to unexpected results – like that it may be an advantage to be exposed to less serious illnesses.
- You might also like: No one wants to foot the bill for new antibiotics
Rare disease can provide greater resistance
“It’s interesting that some people, like those who have the autoinflammatory disease Familial Mediterranean fever (FMF), have mutations in their pyrin molecule. These patients may have an increased level of IL-1β and IL-18,” says Lien.
One hypothesis is that these individuals may have had a different resistance to infections, such as the Black Death, throughout the ages.
Might this population have been more resistant since they have greater amounts of signal substances? Could this have been a positive selection factor that helped them, on average, to have more surviving children than those who did not have increased resistance?
FMF is a rare disease in Norway. It is much more common in Turkey, for example, which was one of the portals for bringing plague from Asia to Europe during the Black Death in the Middle Ages.
“More research may be able to find connections between these pyrin mutations, historic epidemics and pandemics, and modern infections,” Lien says.
He adds that the CEMIR project has already found complex interactions between bacteria and host organisms, and shown new aspects of how the body fights infections.
References:
1. Ratner, D., Ørning, M.P.A., Lien, E. (2016). Bacterial secretion systems and regulation of inflammasome activation. J Leukocyte Biol. Online Nov 3. pii: jlb.4MR0716-330R.
2. Ratner, D., Ørning, M.P.A., Starheim, K.K., Marty-Roix, R., Proulx, M.K., Goguen, J.D., Lien, E. (2016). Manipulation of IL-1b and IL-18 production by Yersinia pestis effectors YopJ and YopM and redundant impact on virulence. J Biol Chem. 291, 9894-905.
3. Ratner, D., Ørning, M.P.A., Proulx, M.K., Wang, D., Gavrilin, M., Wewers, M., Alnemri, E., Johnson, P., Lee, B., Mecsas, J., Kayagaki, N., Goguen, J.D., and Lien, E. (2016): The Yersinia pestis effector YopM inhibits Pyrin inflammasome activation. PLoS Pathogens 12:e1006035.





