How tuberculosis hides in the body
The tuberculosis vaccine only works for children. BCG (bacille Calmette-Guerin) doesn’t protect you as an adult. Now we know more about how the bacterium avoids being detected.
Tuberculosis bacteria hide in the very cells that would normally kill them. Now we know more about how they evade recognition.
You thought you were protected? If you got the BCG vaccine as a child, it has almost certainly stopped protecting you. Around 2 million people die every year from tuberculosis. Photo: Colourbox
Tuberculosis affects millions of people worldwide. Treatment for it is often prolonged, from six months to two years. We thus have a lot to gain — and save — by finding better treatment methods. Improving our understanding about how the bacterium works is key to achieving this.
“Tuberculosis has been around as long as human beings have existed,” says Professor Trude Helen Flo, Co-director of the Centre of Molecular Inflammation Research (CEMIR) at the Norwegian University of Science and Technology (NTNU).
This long history has helped the bacterium to become very widespread. One in every three individuals may be hosting this bacterium, and even if only ten per cent of them contract the illness, it poses a significant problem.
One reason the bacterium has survived for so long is that it has developed a sophisticated mechanism for hiding in the body: it resides in white blood cells called macrophages, the very cells that would normally kill it.
- You might also like: Uncovering the secrets of immune system invaders
Causes infections
Tuberculosis bacteria belong to the mycobacterial pathogens. These mycobacteria can cause chronic infectious diseases.
It can be both dangerous and difficult to study the tuberculosis bacterium, but CEMIR’s new high-risk laboratory now makes such research possible. However, a lot of tuberculosis research is undertaken using the closely related Mycobacterium avium.
This bacterium is far less aggressive and usually only causes problems in birds. It doesn’t kill the cell that it hides in, but is dangerous enough that it can cause infections in individuals with an impaired immune system, such as patients who are receiving cancer treatment, who have pre-existing lung problems or whose immune systems are otherwise compromised.
This makes it interesting to study M. avium directly, but the results can also be transferred to tuberculosis research.
So how do tuberculosis bacteria hide inside the cell?
Confocal microscopy
Confocal microscopy is a technique where laser beams and fluorescence are used to reveal details in a sample. Proteins are either labelled with fluorescent molecules, or fluorescent antibodies recognize the proteins in the sample and bind to them. In either case, the protein will glow a particular colour (wavelength) when illuminated by laser.
The laser only illuminates a small area of the sample at a time. The laser beam is swept across the sample, revealing a thin layer of the cell in two dimensions. Only light from the confocal plane is recorded by detectors. Light above and below that plane (out of focus) is blocked. Three-dimensional images are created by moving the laser beam by degree from the bottom to the top of the cell and depicting a thin layer each time. These images are stacked on top of each other and render a 3-dimensional reconstruction of the cell. This is important because 2-dimensional images can fool you: two objects may lie in the same place in the x-y plane, but far apart in the z-direction, which only appears when the third dimension is depicted.
This type of imaging uses from one to four colours at a time, which works well when the wavelength colours are sufficiently different. That makes it possible to look at the bacteria (which have one colour) and three proteins from the macrophage simultaneously. The researchers take 3D images at different times to increase their understanding of mycobacterial infection inside the macrophage over time and space.
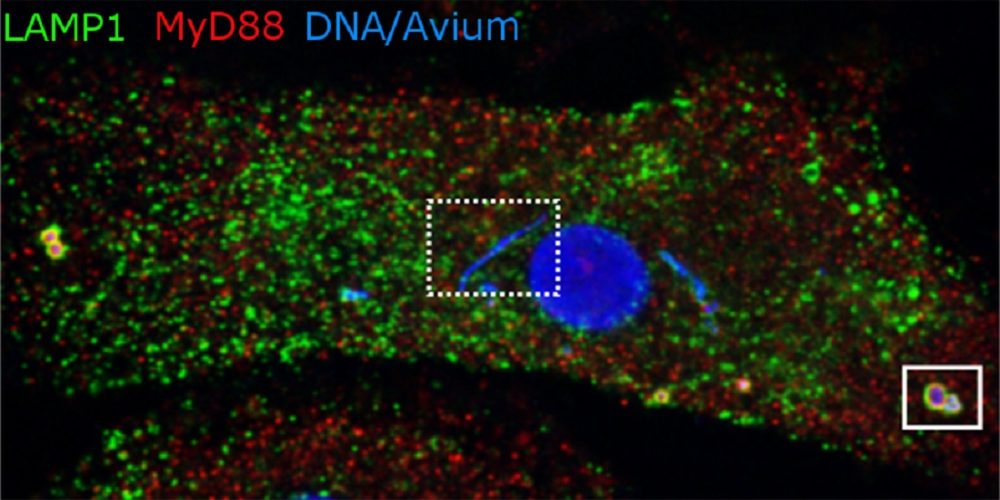
The image shows a macrophage with the mycobacterium M. avium. Bacteria like the one on the right are about to be broken down by lysosomes. But the blue strip in the middle contains bacteria that reside in phagosomes, which for some reason are not being transformed into the degrading lysosomes. See the fact box for more information. Illustration: Alexandre Gidon, CEMIR and Department of Clinical and Molecular Medicine, NTNU
Hidden in the “big eaters”
The mycobacteria are essentially stowaways: they live in macrophages, the major cells in our body whose task is to gobble up different enemies, useless cells and other particles. Macrophages are a kind of brutal guard in the service of our bodies.

From the Coast Hospital in Hagevik in Os. This is where people who had tuberculosis were sent for treatment. Photo: Norwegian National Archives
Most dangerous bacteria are attacked by macrophages. The macrophage engulfs bacteria and immediately traps them in a separate cell compartment, or vesicle. This vesicle is called a phagosome and is the actual “executioner” in the cell.
The phagosome fuses with other vesicles that contain various decomposing substances. It matures and turns into a lysosome. In the lysosome, bacteria are broken into their individual constituents, which can be reused by the body. However, some of the mycobacteria go undetected.
A new study from CEMIR provides new insight into why. The study, led by Professor Flo, has just been published in PLOS Pathogens.
- You might also like: Using Big Data to understand immune system responses
Hiding in a separate compartment
Postdoctoral fellow Alexandre Gidon at CEMIR specializes in the use of advanced microscopes. He and his colleagues have studied mycobacteria and macrophages directly through a confocal microscope. (See fact box.) Gidon is the first author of the new study.
Their research shows that M. avium not only avoids being killed by macrophages, but even avoids being discovered by them. It is this recognition phase that the CEMIR researchers are now able to shed more light on.
What mycobacteria look like inside a cell
- The main image for this article shows blue-stained DNA that marks the nucleus in the centre and mycobacteria in various compartments within the macrophage.
- The mycobacteria located at the edge of the cell are in lysosomes (LAMP1, green colour), where they are broken down. The bacteria that end up in lysosomes are recognized by inflammatory sensors in the macrophage (MyD88 in red). The inflammatory response is important in combating the infection.
- The mycobacteria close to the nucleus are in phagosomes that do not mature to lysosomes (box with dotted line). The inflammatory sensors are either excluded or do not recognize the mycobacteria that grow and divide in these phagosomes.
“How this happens, we don’t yet know. But if we could prevent mycobacteria from hiding in this compartment, or force the bacteria out of it, they would have a hard time not being killed. Then it would have trouble causing a chronic infection,” she said.
An important lead may be that not all mycobacteria manage to avoid being detected. The macrophages engulf all the bacteria, but only the most well-adapted ones are not discovered and manage to hide and survive.
The researchers envision future research where they would try to find out how the mycobacteria manage to establish and sustain the vesicle hideouts as they evade discovery. That would enable something to be done about these chronic infectors.
Better treatment is the goal
The treatment for common tuberculosis usually involves four different antibiotics to start, and gradually decreases to two. The whole process takes about six months. But if the tuberculosis bacterium is resistant to antibiotics, recovery can take up to two years.
“Unlike a number of other infectious diseases, you don’t become immune even if you’ve had tuberculosis. You can become infected and sick again, so it’s important to find solutions that can provide milder, shorter-term treatment,” says Flo.
CEMIR’s basic research is part of this work.
Antibiotics are designed to kill bacteria. Antibiotic resistance is an increasing problem. An effective solution may be to combine antibiotics with substances that stimulate our own immune system. In this way, the bacteria would be attacked from two sides and would therefore have a harder time finding places to hide.
Reference:
Gidon A, Åsberg SE, Louet C, Ryan L, Haug M, Flo TH (2017) Persistent mycobacteria evade an antibacterial program mediated by phagolysosomal TLR7/8/MyD88 in human primary macrophages. PLoS Pathog 13(8): e1006551. https://doi.org/10.1371/journal.ppat.1006551